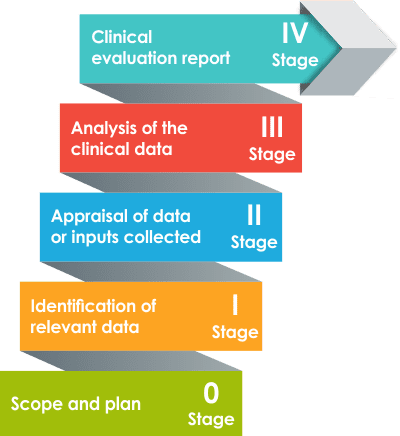
A clinical evaluation plan is a detailed document that outlines the systematic approach and strategy for evaluating the clinical performance and safety of a medical device. It serves as a roadmap for conducting clinical evaluations throughout the device’s lifecycle, from initial development to post-market surveillance.
The clinical evaluation is an essential part of the regulatory submission process, providing evidence to demonstrate the device’s safety, performance, and compliance with regulatory requirements. The purpose of a plan is to gather and assess clinical data to support the device’s safety and efficacy claims.
It involves a comprehensive analysis of existing scientific literature search protocols, clinical investigations, post market surveillance data, and other relevant sources of clinical evidence. The clinical evaluation plan helps establish the scientific validity, clinical performance, and risk-benefit profile of the medical device.
To develop the scope of the medical device clinical evaluation to be conducted, as well as to plan a sound method for identifying, collecting, and analyzing clinical data with the proper schedule and clinical evaluation team.