Performance Evaluation Plan Report for IVDR
A performance evaluation plan report is an important step for in vitro diagnostic devices that serves to meet IVDR EU 2017/746 general safety and performance requirements.
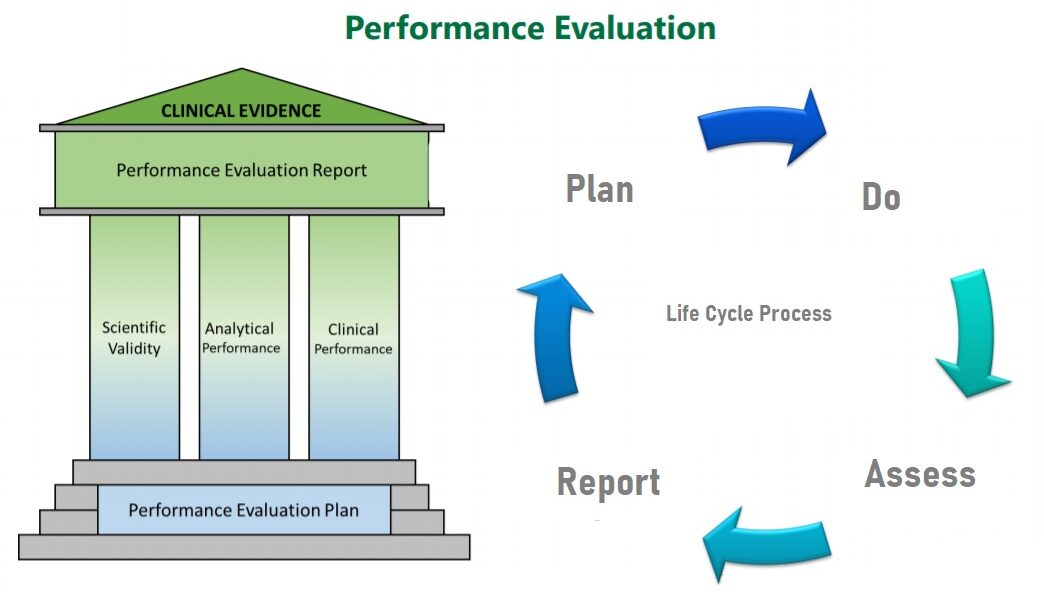
Part A of Annex XIII describes the performance evaluation plan report as a three-step process. Manufacturers are expected to establish, document, and update a performance evaluation plan as a first step. They must also have a SOP template that outlines in full how the scientific validity, analytical, and clinical performance of all of the devices they make will be established.
The Services covered in the Performance Evaluation Plan Report
Performance Evaluation Plan must include the the following:
(a) acceptance criteria for the methods used, and how the acceptability of the benefit-risk ratio will be determined.
(b) conducting the evaluation to collect data, and the
(c) analyzing the data, the conclusions, and the reporting.
The contents of the PEP and PER are clearly defined in Annex XIII.
Some critical points of Performance evaluation report plan are the following.
-
- Intended purpose/intended use
- The analyte or marker
- Target populations
- Description of the state of the art
Data sources for the preparation of the Performance Evaluation Plan
There are various sources and databases available for selecting determining the relevant data for the preparation of the Performance Evaluation Report.
- Data from clinical and analytical performance studies
- Research of scientific literature on Databases like Pubmed, Embase, Cochrane, Medline
- Research on National databases of the competent authorities such as BfArM (Germany), FDA (USA), MHRA (UK)
- Research of ongoing or already completed clinical studies or trials
- Own data resulting from IVD post-market monitoring gathered either actively (user survey) or passively (complaints) in statistically evaluable form.
FAQ's
What is Analytical Performance?
The ability of an IVD device to correctly detect or measure a particular analyte
Analytical performance based on analytical performance studies.
• Should take into account the state of the art.
• Reference materials/methods, where available
• Analytical Performance parameters can be found in IVDR Annex I 9.1.
What are the common Guidelines followed for Performance Evaluation
GHTF/SG5/N6:2012: Clinical Evidence for IVD medical devices – Key Definitions and Concepts
GHTF/SG5/N7:2012: Clinical Evidence for IVD medical devices – Scientific Validity Determination and Performance Evaluation
GHTF/SG5/N8:2012: Clinical Evidence for IVD Medical Devices - Clinical Performance Studies for In Vitro Diagnostic Medical Devices
DIN EN 13612:2002-08: Performance evaluation of in vitro diagnostic medical devices
ISO 20916:2019: In vitro diagnostic medical devices — Clinical performance studies using specimens from human subjects — Good study practice
What are the Notified Body Check Points in a Performance Evaluation Report
Were clinical performance studies and tests performed to prove a device is effective and safe?
What analytical tests were performed and what standards are used?
What recorded evidence exists for the device?
What research has been conducted on the device and it’s intended purpose?
What state-of-art the device corresponds to?
Performance Evaluation for Invitro Diagnostic Medical Devices. What does it mean?
IVDs only need to perform performance evaluations. Clinical Evaluation is used for medical devices. Both words have the same goal: to show that the device complies with the GSPR, however EN13612:2002 for IVDs and UNI EN ISO14155 for other MDs.
Didn't Find the Answer?
Do you wish to know more information performance evaluation plan and report. We will be glad to support you.