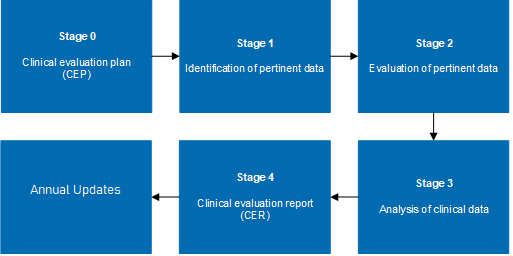
The medical device clinical evaluation process should be planned, conducted, and documented in detail throughout the lifecycle of medical devices and shall be updated if required based on the clinical data obtained from the PMCF plan. Before the medical device is placed on the market and put into service, it should demonstrate conformity with the medical device’s general safety and performance requirements.
The clinical risks shall be addressed as part of clinical investigations, clinical evaluation, and post-market clinical follow-up. The risk management system should be carefully aligned and updated if required. For class III devices and certain class IIb devices, if required, consult an expert panel before the clinical evaluation and investigation. This should be part of the clinical development plan.
If the medical device is a class III and implantable device which have been lawfully placed on the market or put into service in accordance with Directive 90/385/EEC or Directive 93/42/EEC and for which the clinical evaluation: — is based on sufficient clinical data, and — is in compliance with the relevant product-specific CS for the clinical evaluation of that kind of device, where such a CS is available for such devices clinical Investigation is not required.
As per MDR Annex I, conformity with relevant general safety and performance requirements should be set out under the normal conditions of the intended use of the device and evaluate the undesirable side effects and the acceptability of the risk-benefit ratio. This should be documented in the clinical evaluation plan.
The relevant scientific literature currently available relating to the safety, performance, design characteristics, and intended purpose of the device, where it is demonstrated that the device subject to clinical evaluation for the intended purpose is equivalent to the device to which the data related and the data adequately demonstrate compliance with the relevant general safety and performance requirements;
The manufacturer of the equivalent device should have a contract to show for full access to the technical documentation on an ongoing basis to demonstrate the equivalence.
For products without an intended medical purpose, the clinical data concerning safety should be from post-market surveillance, PMCF, and, where applicable, specific clinical investigation. Clinical investigations shall be performed for those products unless reliance on existing clinical data from an analogous medical device is duly justified.
For class III devices and implantable devices, the PMCF evaluation report, and the summary of safety and clinical performance shall be updated at least annually. The output of the medical device clinical evaluation report should be updated in Summary of Safety and Clinical Performance (SSCP) and Risk Management.