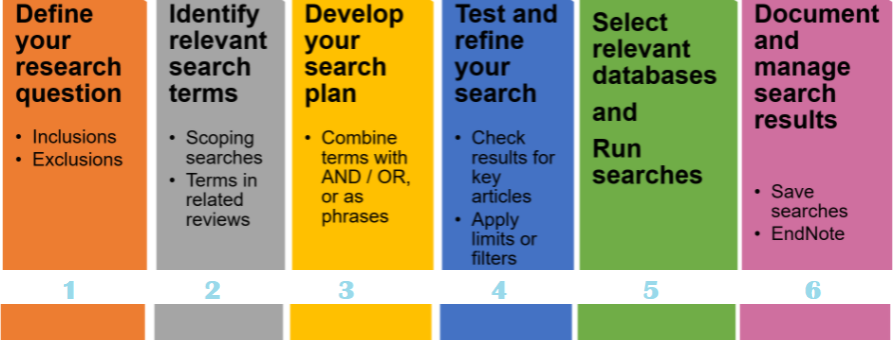
Literature search protocol for medical devices should be developed and executed by identified persons with expertise in information retrieval, having due regard to the scope of the clinical evaluation.
To plan and keep a record of the identification and review of clinical data, conduct a literature search in response to review questions on safety, performance, undesirable side effects, and risk-benefit analysis. The scope is limited to medical devices planned for a clinical evaluation using data obtained from works of literature.
The results of the Literature search protocol and conclusion should include the data derived from each piece of literature and its relevancy to answer the review question. If the data is not enough to conclude, it should be justified, and more data requirements should be specified.
Scientific Literature Search Protocol and Review Services
We have developed useful Literature search protocol templates and ready for online buying.
CONTACT US
Importance of Literature Search Protocol
Because a robust and systematic literature search approach fortifies every stage of the medical device life cycle process from idea and design, literature reviews are critical to the success of clinical evaluation report documentation. As a result, screening the literature to ensure compliance with the CE Notified Body throughout the approval process and for post-market surveillance is critical to the success of any Medical Device CE Marking.
For many small and medium-sized device makers, the data received via medical device literature search protocol will constitute the majority, if not the entirety, of the data collected. As such, this search discovers clinical data sources for establishing current knowledge or “the-state-of-the-art” that describes the clinical background in the appropriate medical sector, clinical data relevant to the device under assessment, or an analogous device. That is why it is crucial to build a comprehensive literature search method that can be repeated by anybody throughout subsequent upgrades.
FAQ's
Explain Clinical Evidence
Clinical data and clinical evaluation results pertaining to a device of a sufficient amount and quality to allow a qualified assessment of whether the device is safe and achieves the intended clinical benefit(s), when used as intended by the manufacturer.
Explain Clinical Performance
The ability of a device, resulting from any direct or indirect medical effects which stem from its technical or functional characteristics, including diagnostic characteristics, to achieve its intended purpose as claimed by the manufacturer, thereby leading to a clinical benefit for patients, when used as intended by the manufacturer.
Explain Clinical Data
Information concerning safety or performance that is generated from the use of a device and is sourced from the following:
• clinical investigation(s) of the device concerned
• clinical investigation(s) or other studies reported in scientific literature, of a device for which equivalence to the device in question can be demonstrated
• reports published in peer reviewed scientific literature on other clinical experience of either the device in question or a device for which equivalence to the device in question can be demonstrated
• clinically relevant information coming from post-market surveillance, in particular the post-market clinical follow-up