EU 2017/745 enforces all manufacturers must clinically evaluate a medical device before they apply with any Notified Body or placing it on the European market. To do so, the manufacturer has to collect and evaluate clinical data in order to investigate whether the medical device is safe and performing. However, if the clinical data are insufficient, manufacturer must conduct medical device clinical trials also know as “clinical investigations”.
Clinical Trials
EU 2017/745, the requirements for medical device clinical trials are part of Art. 62 through 82 and Annex XV.
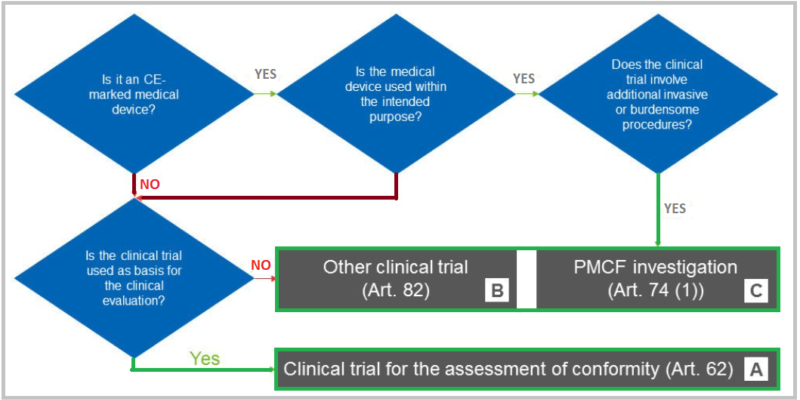
Types of Medical Device Clinical Trials
FAQ's
How to conduct medical Device Clinical Trials
Will be updated soon
Data Protection and Medical Device Clinical Trials
Will be updated soon
Application Documents
Will be updated soon
Role of Sponsor
Will be updated soon
Ethics Committee
Will be updated soon
Reduced burden for your employees
Contact us with your plans or ask us more information